Evidence-Based Vaccine Recommendations for Dermatology Patients
Megan H. Noe, MD, MPH, MSCE
Assistant Professor of Dermatology, Brigham and Women’s Hospital, Harvard Medical School
August 2023
Dr. Noe presented an overview of vaccine recommendations and considerations for dermatologists. Although vaccines are typically discussed in primary care settings, dermatologists have an important role in vaccine education, especially when prescribing immunosuppressive medications that can increase risk of infections. Infections are more common in patients with chronic skin diseases due to disruption of the protective skin barrier, immune dysregulation associated with disease, and immunosuppressive treatments.
Dr. Noe discussed 3 common clinical scenarios where dermatologists can incorporate vaccine education into patient care. She suggests taking vaccine history and recommending any missing vaccines before prescribing immunosuppressive medications.
Case #1
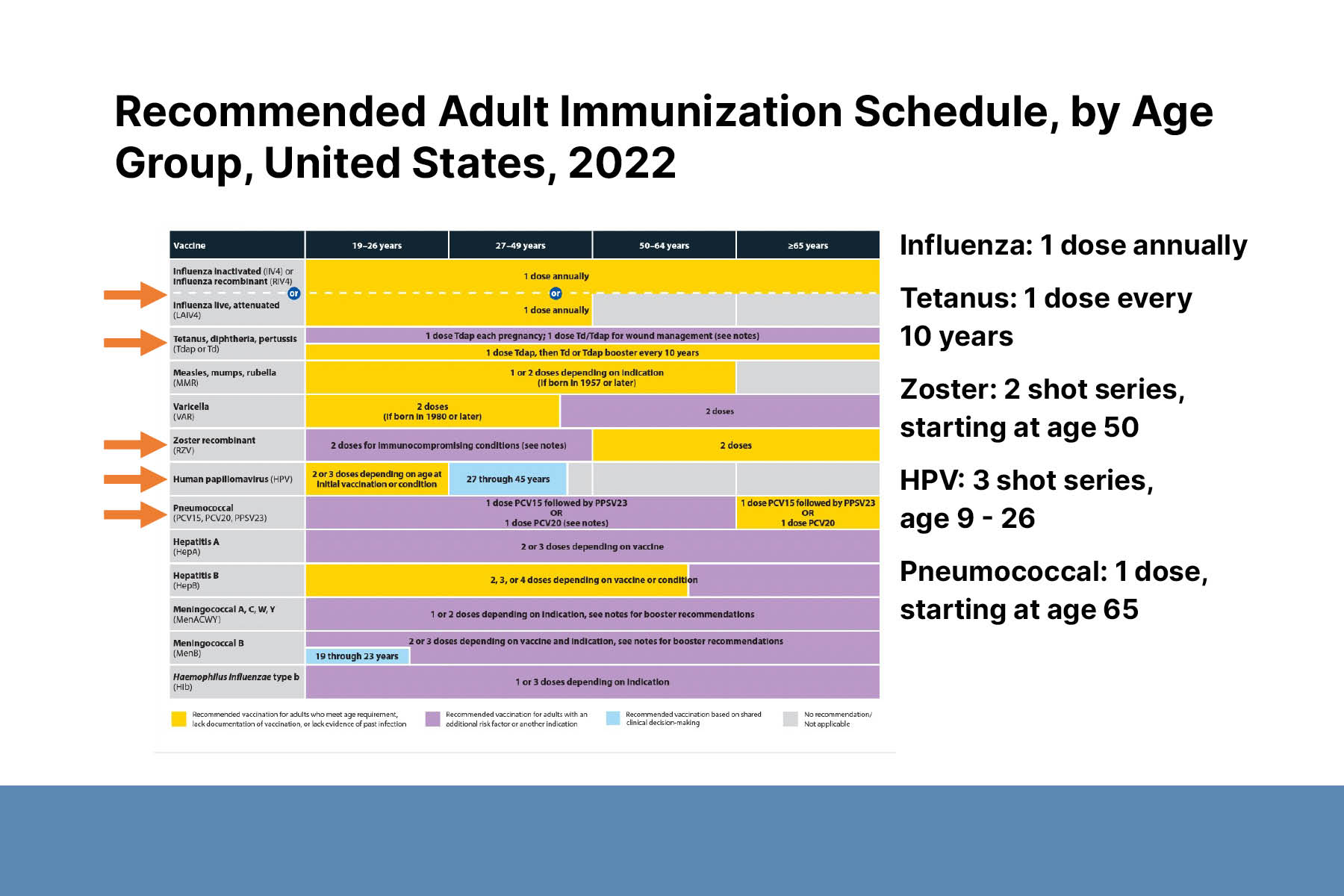
A 45-year-old with atopic dermatitis will start treatment with upadacitinib, a selective JAK1 inhibitor. JAK inhibitors can increase the risk of infections, especially shingles, caused by the varicella zoster virus. Thus, dermatologists should recommend the herpes zoster vaccine to patients before starting JAK inhibitors. SHINGRIX, an FDA-approved herpes zoster vaccine, is a 2-dose series given 2–6 months apart. It is indicated for everyone >50 years and immunocompromised adults ≥19 years. It is also recommended for patients who previously received the live vaccine. While taking JAK inhibitors, patients can continue to receive all recommended vaccinations, including annual flu shot, but live vaccines are contraindicated.
Case #2
A 50-year-old with a history of pemphigus foliaceous will start a follow-up course of rituximab after a flare. Rituximab increases the risk of infections up to 12 months after treatment. The risk of serious infection increases with additional treatment cycles. Rituximab impairs response to vaccinations during treatment and for ≥6 months afterwards. All patients should receive pneumonia, shingles, flu, and COVID vaccines ≥ 3–4 weeks before starting treatment. To determine when a patient can receive a vaccine after stopping treatment, consider checking CD19+/CD20+ cell levels to monitor B-cell repopulation.
Case #3
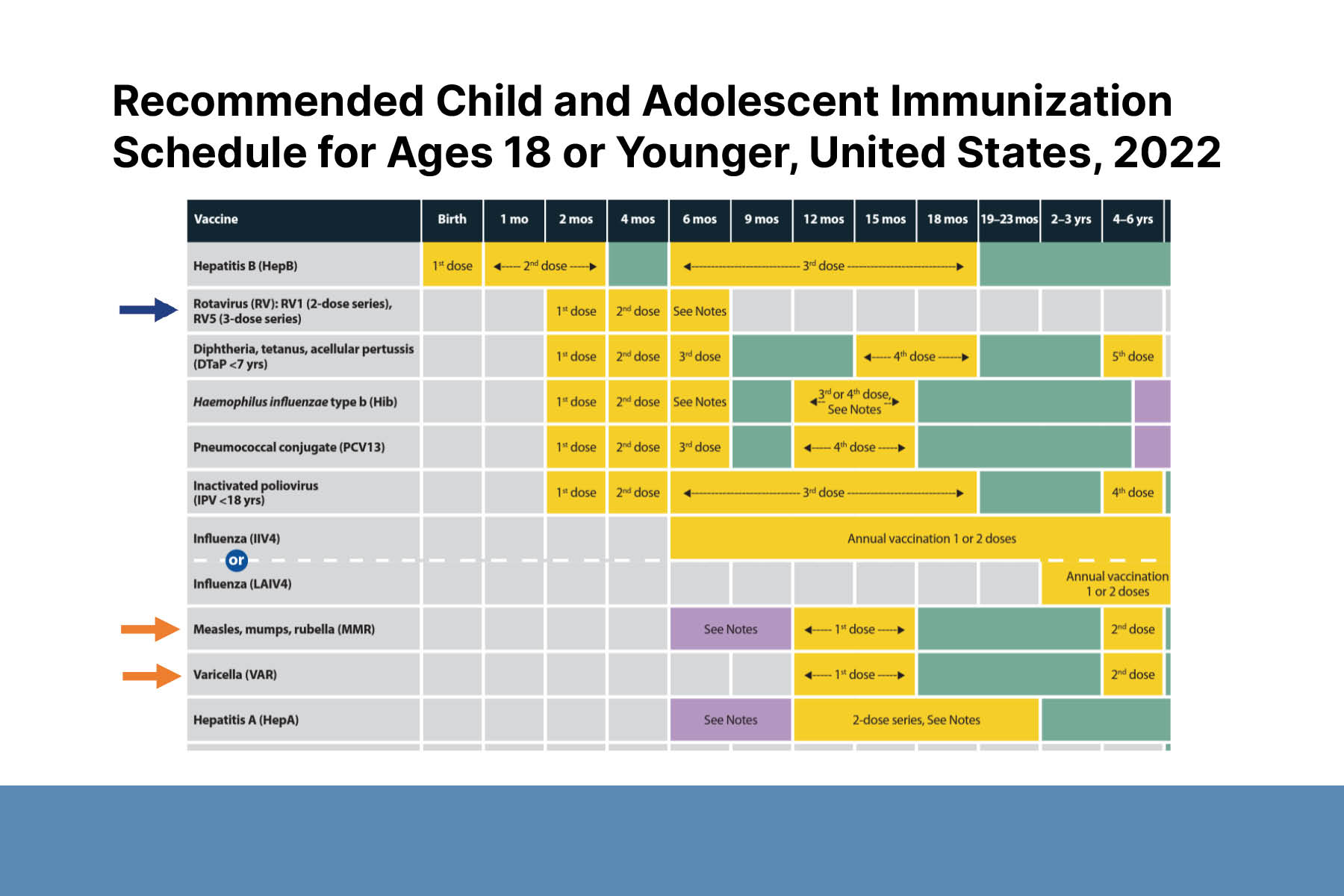
A 12-month-old with severe atopic dermatitis will start FDA-approved dupilumab. Live vaccines are contraindicated in all patients taking dupilumab. This is especially significant for children because important childhood vaccines (e.g., rotavirus, measles, mumps, and rubella; and varicella) are live vaccines. The need for live vaccinations should be taken into consideration before starting treatment. Studies and anecdotal evidence in adults show dupilumab may not significantly impair immune response to vaccines. More research is underway to improve clinical guidance in the pediatric population.