In-Transit Melanoma Metastases
Christian Baum, MD
Professor and Vice-Chair of Dermatology, Mayo Clinic Rochester, Minnesota
December 2023
Dr. Baum described melanoma in-transit metastases (ITMs) and presented an overview of current treatments. Melanoma ITMs are lesions that develop between the primary tumor site and the draining nodal basin via lymphatic seeding, separate from spreading in the blood. ITMs occur in 5–10% of patients with melanoma and typically develop within 13–18 months. Fifty percent of patients with melanoma ITMs develop melanoma metastases in other organ systems in the body. Historically, patients with melanoma ITMs have not been included in clinical trials or national registries.
Melanoma ITMs are at least AJCC Stage III regardless of nodal involvement. NCCN recommends imaging for baseline staging in Stage III disease. Sentinel lymph node biopsy (SLNB) for resectable ITMs may be considered even if already performed for the primary tumor. SLNB may be more precise than CT scans or physical examination.
Treatments for melanoma ITMs include surgery, isolated limb perfusion (ILP), radiation, intralesional therapy, and topical therapy. Surgery is a reasonable first option if there is no nodal involvement. Radiation can provide local control, but lesions may form outside the treated area. ILP uses high-dose chemotherapy and can be well tolerated, even in patients older than 70 years. Intralesional therapies include Bacillus Calmuette-Guérin, interleukin-2, rose bengal, and talimogene laherparepvec. Interleukin-2 is FDA-approved for Stage IV melanoma. Talimogene laherparepvec, an oncolytic virus, is FDA-approved for unresectable lesions recurrent after surgery.
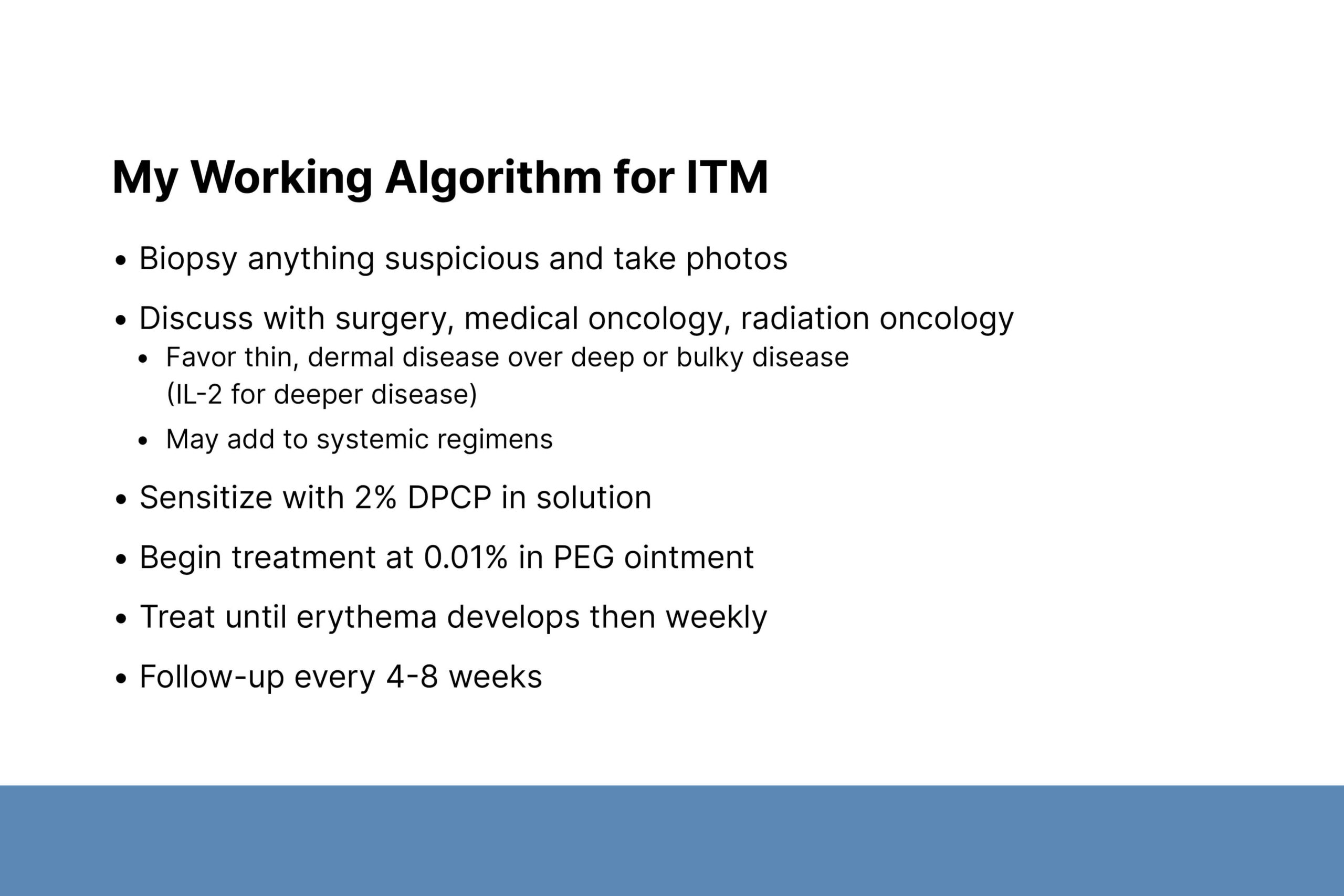
Dr. Baum discussed the advantages of topical therapy for melanoma ITMs and encouraged its use as part of a multidisciplinary approach. Topical therapy costs less, is easy to apply, and gives patients an active role in fighting the disease. Topical therapies include azelaic acid, miltefosine, imiquimod, 5-fluorouracil, dinitrochlorobenzene, and diphenylcyclopropenone (DPCP). DPCP has been evaluated in the largest number of studies. In a study of 58 patients with melanoma ITMs, 50% of patients showed a treatment response; 15–20% had progressive disease.
Several of Dr. Baum’s patients were successfully treated with DPCP. Typically, he treats patients with superficial/non-bulky disease using a 2% solution to sensitize the skin, followed by 0.01% DPCP ointment. Patients with deeper disease are discussed with medical and radiation oncology teams for treatment options. The Mayo Clinic has an open clinical trial for patients with melanoma ITMs to better understand the disease.