
Twenty-two years ago, Kurt Lu, MD, was a postdoctoral fellow in molecular and cellular dermatology at Case Western Reserve University in Cleveland. He was studying macrophages with Kevin Cooper, MD and wanted to know more about the role these cells play in the resolution of inflammation in the skin.
“Macrophages are in every organ system, where they respond to injury and infection,” Lu said. “They’re also important in the resolution of inflammation but, at the time, not much was known about how that worked.”
Emerging data from the immunology field identified two subsets of macrophages involved in inflammation: M1 macrophages are pro-inflammatory, while M2 are anti-inflammatory. Early in his junior faculty years, he worked with Roy Silverstein, MD and Mukesh Jain, MD. His studies led to a better understanding of how these cell populations may be involved in the resolution of inflammation in the skin. Along with his colleagues, Lu published an early study elucidating the factors involved in determining macrophage state and how cells can undergo polarization to one subset or the other (Liao et al. 2011).
Gene expression differs between the M1 and M2 subsets, including that of a critical regulatory molecule, Krüppel-like factor 4 (KLF4), which is needed for polarization of macrophages to the M2 state. In KLF4 knockout mice, injured skin continued to break down because M1 macrophages could not become anti-inflammatory. M1 cells were also characterized by elevated levels of inducible nitric oxide synthase (iNOS), while M2 cells had high levels of IL-10.
“This research fueled our strategy to find ways to tweak cells to become high-IL-10 proresolutory macrophages,” Lu said. “The obvious question was whether we could find pharmacologics that would drive this polarization and, perhaps, resolve inflammatory skin diseases.”
His journey since then has converged on the discovery and testing of translational drugs to treat skin injury and inflammation. It has led to two naturally-occurring compounds that tell a coherent story — vitamin D and melanin.
A scientist from an early age
Lu grew up in New York City and went to Bronx High School of Science. He was a Westinghouse Scholar for three years, conducting research at Rockefeller University under the tutelage of Steven Morris, PhD with whom he studied immunology and virology. Lu received his medical degree from University of Rochester School of Medicine, where he worked with the renowned pediatric immunologist, Richard Insel, MD, for six years.
He received his first Dermatology Foundation award, a fellowship, in 2007. After completing his residency and postdoc at Case Western, Lu became an attending physician and assistant professor. During this time, the Foundation awarded him a Career Development Award (2008–2010).
“The CDA allowed me to continue my work in macrophage biology, inflammation, and wound healing,” Lu said. “This funding helped launch my career, leading to multiple NIH awards and a center grant.”
After achieving associate professor with tenure, he moved to Northwestern University Feinberg School of Medicine in Chicago. Since 2018, he is the Eugene and Gloria Bauer professor of dermatology where he practices dermatology and conducts fundamental and translational research to discover and develop therapeutics for skin and wound repair.
Lu has been a long-time supporter of the Dermatology Foundation and a member of the Leader Society since December 2012.
Vitamin D affects macrophage polarization and has a positive effect on skin injury
When Lu was investigating the causes of macrophage polarization in the early 2000s, there was widespread interest in the role of vitamin D in health and it emerged that vitamin D was a critical immune modulator, specifically in macrophages. Vitamin D was known to lower iNOS levels and increase those of IL-10, piquing his interest since these were characteristics of M2 macrophages. Lu had recently attended a conference at which Rebecca Boxer MD, presented heart failure study results showing a lowering of certain inflammatory cytokines following vitamin D treatment. Lu wondered if the inflammatory cytokines were derived from macrophages and whether vitamin D was capable of pushing macrophages towards the M2 population. He went back to his lab and devised experiments to test this.
Since macrophages play a key role in homeostasis, Lu provoked these cells by designing a tissue injury model using the PPAR-gamma agonist rosiglitazone, a diabetes drug. The unexpected finding of a drug hyper-induced iNOS and led to M1 polarization (Das et al. 2015). These M1 macrophages caused de novo tissue damage, providing a testable model of a chronic wound.

Co-corresponding authors Nathan Gianneschi, PhD (left) and Dr. Kurt Lu (right). Photo credit: Kristin Samuelson, Northwestern University.
Lu was ready to test vitamin D as a treatment for skin damage. But at what dose? A drug like ibuprofen at 200-400 mg can modulate some pain, but it takes 600 mg, four times a day to see anti-inflammatory effects. Typically, vitamin D is dosed up to only 2,000 IU as a supplement. Perhaps much more was needed to see an anti-inflammatory response.
Higher doses were thought to be safe for two reasons. First, the body regulates the amount of vitamin D in the bloodstream. Oral doses of vitamin D undergo first-pass metabolism, with primary hepatic hydroxylation to 25-hydroxyvitamin D, then a secondary hydroxylation in the kidney to convert it to its active form. Second, the VIOLET trial had already demonstrated a lack of toxicity of an enteral dose of 540,000 IU of vitamin D (National Heart, Lung, and Blood Institute PETAL Clinical Trials Network et al. 2019).
Lu and his colleagues completed a dose-finding study with the most straightforward inducer of skin injury: sunburn. The resulting double-blind, placebo-controlled pilot study found that increasing doses up to 200,000 IU attenuated skin burn (Scott et al. 2017). At that higher dose, study participants with a demonstrable elevation of vitamin D in serum had the greatest suppression of erythema. Skin biopsies following high doses revealed lower expression of TNF-ɑ and iNOS, and increased expression of arginase-1, an important enzyme that processes arginine to become either iNOS or proceed down an anti-inflammatory pathway.
How does vitamin D mitigate inflammation?
To complete the story, Lu wanted to understand how vitamin D was leading to an increase in arginase-1 expression. Using molecular, cellular, and knockout studies in an animal model, Lu and colleagues demonstrated that vitamin D induced autophagy in macrophages, recycling organelles to create energy for the transition to proresolutory M2 cells (Das et al. 2019). It can do this because macrophages are not dependent on renal hydroxylation to convert vitamin D to its active form, instead using intracellular 1,25-hydroxylase to convert to the active form of vitamin D.
Video courtesy of Northwestern University.
“Vitamin D promotes cell renewal,” Lu said. “We couldn’t design a drug better than this. First, the drug has regulatory steps in the kidney and liver that prevent systemic toxicity, even at high doses. But it specifically targets pathologic M1 cells producing a lot of iNOS at the site of injury that are leading to continued breakdown of tissue. And these cells are responsive to the drug, and don’t require activation via gatekeeper steps from the kidney.”
Clinical applications of vitamin D in dermatology
Lu and colleagues tested whether insights from UV-induced skin injury applied to chemical injury using skin damage from a chemotherapeutic alkylating agent for cutaneous T-cell lymphoma (CTCL) as a model. While chemotherapy provided a great clinical response, skin pain for some patients became so intense that they wanted to stop treatment. Skin biopsies of those with skin pain showed high levels of iNOS M1 macrophages, suggesting that injury attracted active M1 cells to the skin (Tacastacas et al. 2017). High-doses of vitamin D reduced the skin pain and patients were able to continue topical chemo treatments.
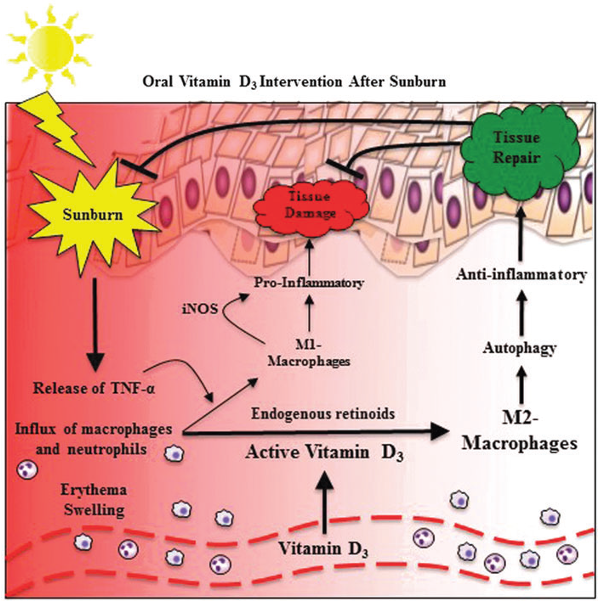
Ultra-high doses of vitamin D lead to increased concentrations of inactive vitamin D in circulation. Macrophages convert this to the active form, which promotes the differentiation to M2 proresolutory macrophages containing arginase-1 and evidence of autophagy. Thus, ultra-high doses of vitamin D eliminate the inflammation caused by sunburn (From Scott and Lu, 2017). Copyright © 2017, Mary-Ann Liebert, Inc. Reprinted with permission.
Lu turned to mouse models to investigate what was happening. This culminated in a study, similar to the sunburn study, showing the positive effects of 200,000 IU vitamin D on skin damaged by chemical exposure (Ernst et al. 2023). He and his team have since shown that high-dose vitamin D aids in the management of toxic erythema of chemotherapy (Nguyen et al. 2023a), acute radiation dermatitis (Nguyen et al. 2023b), and chemical and radiation-induced skin injury during cancer therapy (Nguyen et al. 2024).

Co-corresponding author Nathan Gianneschi, PhD speaks to a member of his lab on Northwestern University’s campus. Photo credit: Kristin Samuelson, Northwestern University.
The clinical implications of this are clear — vitamin D might prove to be a good treatment for inflammation caused by sunburn and other sources of skin injury (Scott and Lu, 2017). Lu and his team selected this safe and affordable compound for these proof-of-concept studies. While clinical studies are needed to confirm the benefits of single high-dose treatments for skin injury, excessive vitamin D supplementation poses health risks (NIH 2025). High-dose vitamin D use remains off-label and unapproved by the FDA, though there are anecdotes of off-label use after excessive sun exposure (McGrath and Lu 2025).
Synthetic melanin as a novel approach
Melanin, which is conserved across evolutionary lines, is another molecule known to protect skin from UV radiation, by absorbing light and scavenging reactive oxygen species (ROS) that can lead to inflammation and subsequent skin degradation and cancer.

Co-corresponding authors Dr. Kurt Lu (left) and Nathan Gianneschi look at a sample in Lu’s dermatology lab at Northwestern University.
Photo credit: Kristin Samuelson, Northwestern University.
Lu has collaborated with nanotechnologists at Northwestern University, including Nathan Gianneschi, PhD to develop an engineered compound to mimic one form of melanin linked together to form synthetic melanin particles. When applied in a topical skin cream, synthetic melanin leads to increased levels of the skin’s inherent superoxide dismutase without being absorbed and accelerates tissue healing in mice and human skin explant models (Biyashev et al. 2023; Lu and Gianneschi 2025). Melanin absorbs ROS, which dampens inflammation of the epidermis and creates a favorable environment for the polarization of macrophages from the M1 to M2 subset (Biyashev et al. 2023).
“It acts like a molecular vacuum, absorbing inflammatory factors in addition to being a free radical scavenger,” Lu said. “Synthetic melanin doesn’t stop the immune cells from coming into the skin. Instead, it modifies the skin environment to one favorable for a switch to the M2 class.”
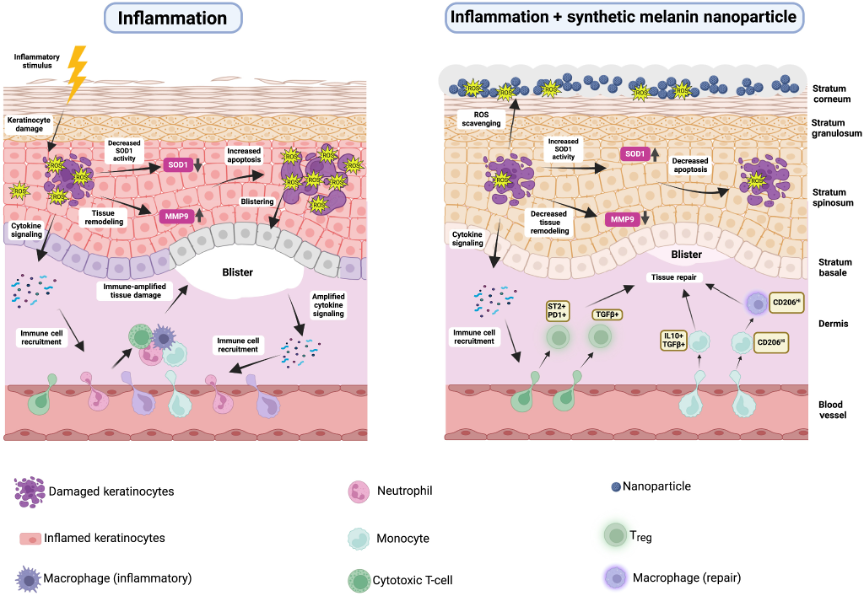
From Lu and Gianneschi (2025). Copyright © 2025 The Authors. Published by Elsevier, Inc. on behalf of the Society for Investigative Dermatology. Reprinted with permission.
Lu and Gianneschi co-founded Melanyze, a company that has designed synthetic melanin to expand the molecule’s applications in dermatology. Synthetic melanin particles are currently in the early drug development stages with the goal to advance the novel active compound for mitigating damage from sunburn, radiation therapy, and chemical burns.
“Understandably, inflammatory skin diseases like psoriasis and atopic dermatitis are often top of mind in dermatology research,” Lu said. “I’d like to see more research focusing on skin injury as clinically we have limited treatment options beyond supportive care. The inflammatory factors involved, the models we use, and the way this translates to treating different skin conditions are similar.”
The hope is this work, and research like it, will yield clinically relevant treatments in the near future.
References
Biyashev D, Siwicka ZE, Onay UV, et al. Topical application of synthetic melanin promotes tissue repair. NPJ Regen Med. 2023;8(1):61. https://www.nature.com/articles/s41536-023-00331-1
Das LM, Rosenjack J, Au L, et al. Hyper-inflammation and skin destruction mediated by rosiglitazone activation of macrophages in IL-6 deficiency. J Invest Dermatol. 2015;135(2):389–399.https://pubmed.ncbi.nlm.nih.gov/25184961/
Das LM, Binko AM, Traylor ZP, et al. Vitamin D improves sunburns by increasing autophagy in M2 macrophages. Autophagy. 2019;15(5):813–826. https://www.tandfonline.com/doi/full/10.1080/15548627.2019.1569298
Ernst MK, Evans ST, Techner JM, et al. Vitamin D3 and deconvoluting a rash. JCI Insight. 2023;8(2): e163789. https://insight.jci.org/articles/view/163789
Liao X, Sharma N, Kapadia F, et al. Krüppel-like factor 4 regulates macrophage polarization. J Clin Invest. 2011;121(7):2736–2749. https://www.jci.org/articles/view/45444/pdf
Lu KQ, Gianneschi NC. Synthetic Melanin as a Topical Agent for Accelerated Skin Repair. J Invest Dermatol. 2025;145(2):237–239. https://www.jidonline.org/article/S0022-202X(24)01981-X/fulltext
McGrath JA, Lu KQ. Single high-dose vitamin D3: a promising sunburn therapy. Br J Dermatol. 2025;192(2):181–182. https://academic.oup.com/bjd/article/192/2/181/7756515?login=false
National Heart, Lung, and Blood Institute PETAL Clinical Trials Network; Ginde AA, Brower RG, Caterino JM, et al. Early High-Dose Vitamin D3 for Critically Ill, Vitamin D-Deficient Patients. N Engl J Med. 2019;381(26):2529–2540. https://www.nejm.org/doi/10.1056/NEJMoa1911124?utm_source=chatgpt.com
NIH Office of Dietary Supplements. Vitamin D: Fact sheet for health professionals. Accessed 26 March 2025. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/#en158
Nguyen CV, Zheng L, Zhou XA, Ernst MK, Kye Y, Choi JN, Lu KQ. High-Dose Vitamin D for the Management of Toxic Erythema of Chemotherapy in Hospitalized Patients.
JAMA Dermatol. 2023a;159(2):219–222. https://jamanetwork.com/journals/jamadermatology/fullarticle/2799537
Nguyen CV, Zheng L, Lu KQ. High-dose vitamin D for the management acute radiation dermatitis. JAAD Case Rep. 2023b;39:47–50. https://jaadcasereports.org/retrieve/pii/S2352512623002412
Nguyen CV, Lu KQ. Vitamin D3 and its Potential to Ameliorate Chemical and Radiation-Induced Skin Injury During Cancer Therapy. Disaster Med Public Health Prep. 2024 Jan 15;18:e4. https://www.cambridge.org/core/journals/disaster-medicine-and-public-health-preparedness/article/vitamin-d3-and-its-potential-to-ameliorate-chemical-and-radiationinduced-skin-injury-during-cancer-therapy/E76A06D85F1BB25694EC693E61334241
Scott JF, Das LM, Ahsanuddin S, et al. Oral Vitamin D Rapidly Attenuates Inflammation from Sunburn: An Interventional Study. J Invest Dermatol. 2017;(10):2078–2086. https://www.jidonline.org/article/S0022-202X(17)31558-0/fulltext
Scott JF, Lu KQ. Vitamin D as a Therapeutic Option for Sunburn: Clinical and Biologic Implications. DNA Cell Biol. 2017;36(11):879–882. https://www.liebertpub.com/doi/10.1089/dna.2017.3978
Tacastacas JD, Chan DV, Carlson S, et al. Evaluation of O6-Benzylguanine-Potentiated Topical Carmustine for Mycosis Fungoides: A Phase 1-2 Clinical Trial. JAMA Dermatol. 2017;153(5):413–420. https://pmc.ncbi.nlm.nih.gov/articles/PMC5817489/

