Oncodermatopathology Clues: Clinical-Pathologic Correlation for Adverse Skin Reactions to Newer Oncologic Therapies
July 2025
Dr. Emily Chu presented information about cutaneous adverse reactions to targeted oncologic therapies, including programmed cell death protein 1 (PD-1)/programmed cell death-ligand 1 (PD-L1) inhibitors, enfortumab vedotin (EV), v-raf murine sarcoma viral oncogene homolog B1 (BRAF) inhibitors, and mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (MEK) inhibitors. As more patients receive these oncologic therapies, dermatologists are treating an increasing number of patients with cutaneous adverse reactions. These reactions are often predictable based on the mechanism of action of the targeted therapy. Certain histopathologic findings are also helpful in establishing these diagnoses.
First, Chu described cutaneous adverse reactions seen in patients receiving PD-1/PD-L1 inhibitors. PD-1 is a surface receptor expressed by T cells. It promotes antigen-specific T cell apoptosis and reduces regulatory T cell apoptosis by interacting with its ligand, PD-L1. Tumor cells that express PD-L1 can escape immune system detection. PD-1/PD-L1 inhibitors boost immunity and promote T cell activation and survival by preventing PD-1/PD-L1 interaction.
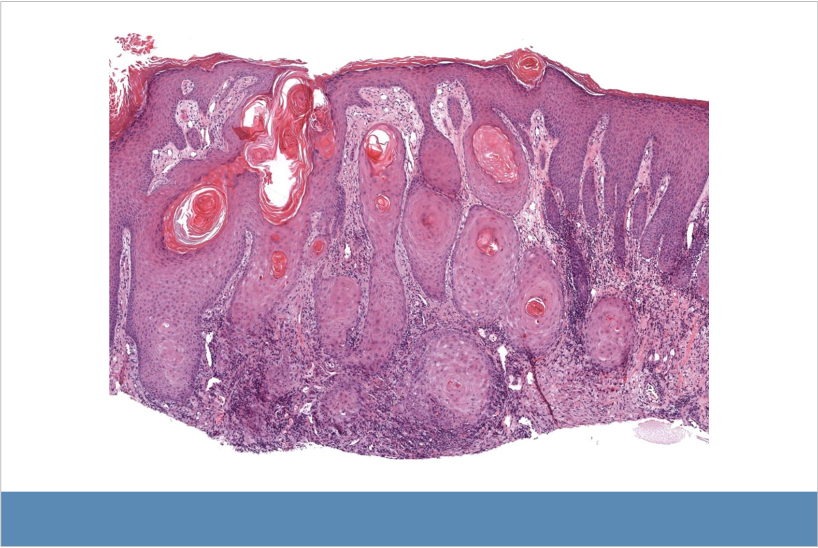
Atypical squamous proliferation associated with PD-1 inhibitor. Lichenoid inflammation is apparent at bottom of image.
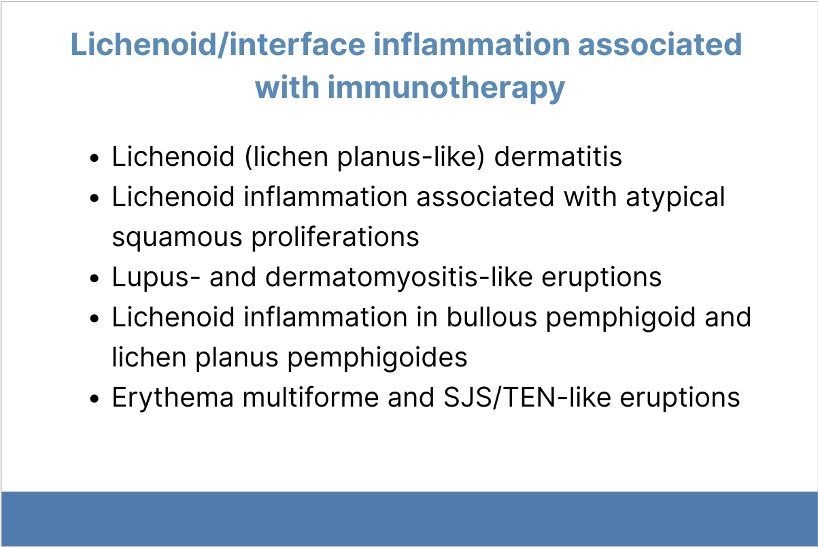
Patients who receive immune checkpoint inhibitors (ICIs), including PD-1/PD-L1 inhibitors may present with hypertrophic lichenoid reactions and squamous cell carcinoma/keratoacanthoma. PD-1/PD-L1 inhibitor therapy is associated with lichenoid and lichenoid interface inflammation, including lichenoid dermatitis, lichenoid inflammation associated with atypical squamous cell proliferation, lupus- and dermatomyositis-like eruptions, PD-1/PD-L1 inhibitor-associated bullous pemphigoid and lichen planus pemphigoides, and erythema multiforme- and Stevens-Johnson syndrome/toxic epidermal necrolysis-like eruptions. Clinical-pathologic correlation is especially important in these cases.
Deeper biopsies may be needed for accurate diagnosis, particularly in differentiating between hypertrophic lichen planus-like reactions and squamous cell carcinoma/keratoacanthoma. Adverse reactions to PD-1/PD-L1 inhibitors may be delayed up to 3 months after starting treatment and can occur even after the treatment is discontinued.
Second, Chu discussed cutaneous adverse reactions seen in patients who receive EV. EV is an anti-nectin-4 antibody conjugated to monomethyl auristatin E (MMAE), which is indicated for advanced urothelial carcinoma after disease progression while taking platinum-based chemotherapy and PD-1/PD-L1 inhibitors. Nectin-4 is a tumor-associated antigen normally expressed in the skin and overexpressed in lung, breast, gastric, and urothelial cancer cells.
EV binding to nectin-4 in the skin may result in keratinocyte microtubule disruption by MMAE that mimics the mechanism of action of taxane chemotherapies. EV-associated eruptions have similar histopathologic features to taxane-induced toxic erythema of chemotherapy, including ring or starburst mitotic patterns, interface dermatitis, and epidermal dysmaturation. Patients who received prior ICI treatment may have more severe EV-associated cutaneous adverse reactions.
Third, Chu described cutaneous adverse reactions seen in patients who receive BRAF and MEK inhibitors. These therapies target the MAPK signaling pathway, which is overactivated in certain cancers. Cutaneous adverse reactions associated with BRAF inhibitors include verrucous keratoses, acantholytic dyskeratosis, keratosis-pilaris like eruptions, new or darkening nevi, and palmoplantar keratoderma; straight hair may become curly. These changes are also seen in inherited melanoma syndromes caused by BRAF and MEK mutations, including cardiofaciocutaneous syndrome and Costello syndrome. Cutaneous adverse reactions associated with BRAF inhibitors are less common with dual BRAF/MEK inhibitor therapy.
To conclude, Chu discussed research on cutaneous adverse reactions to BRAF and MEK inhibitors that provide insight into the pathophysiology of skin diseases and may lead to therapeutic advances.
Mark January 28-31, 2026 on your calendar for the 2026 Annual DF Clinical Symposium.

