Off to the Races: Engineering CA(A)R-T Cells for Skin Disease Therapy
Aimee S. Payne, MD, PhD
Professor of Dermatology, University of Pennsylvania
August 2023
Dr. Payne presented ongoing research into chimeric antigen receptor (CAR)-T cell therapy and chimeric autoantibody receptor (CAAR)-T cell therapy for cancers and autoimmune diseases, including pemphigus vulgaris (PV).
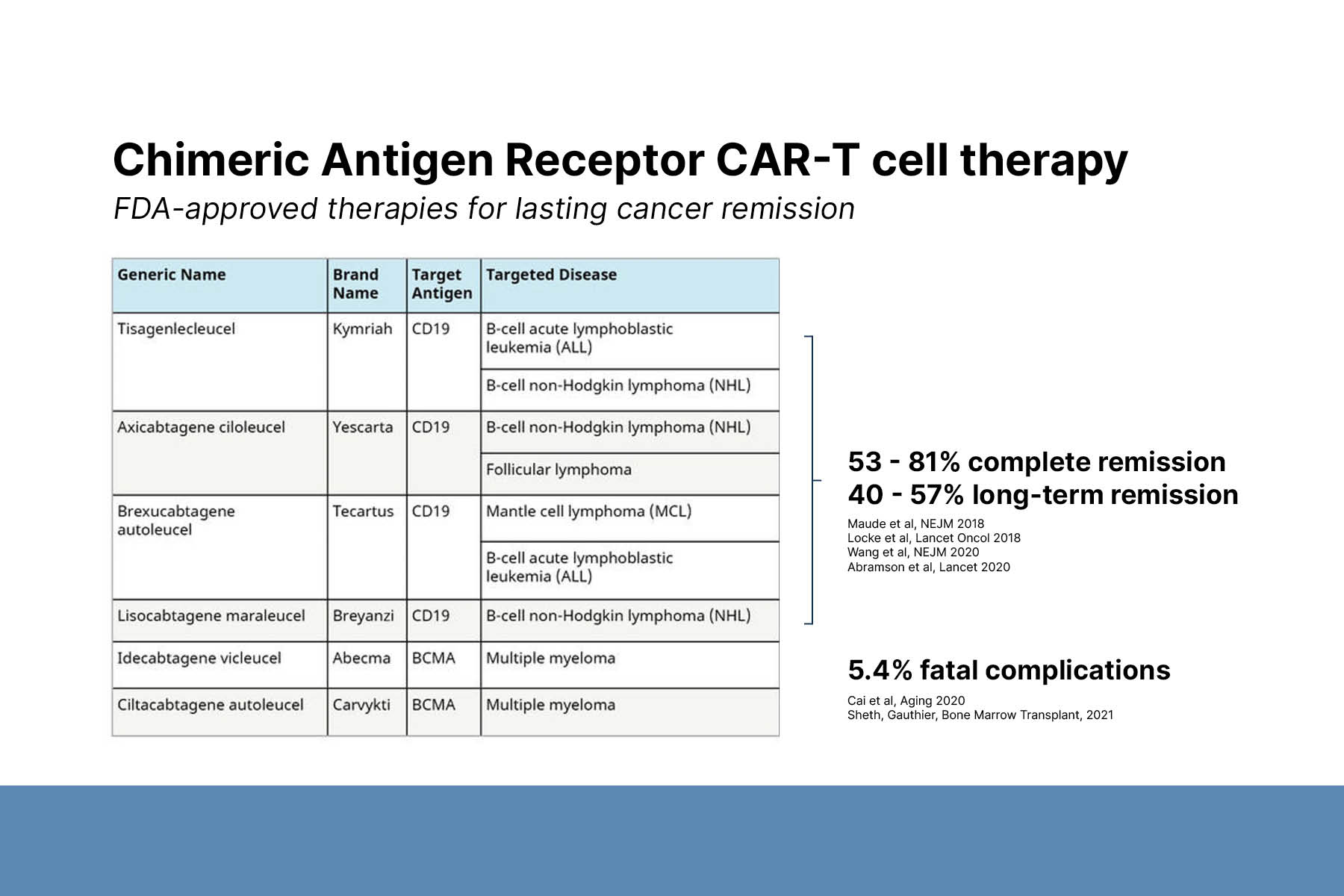
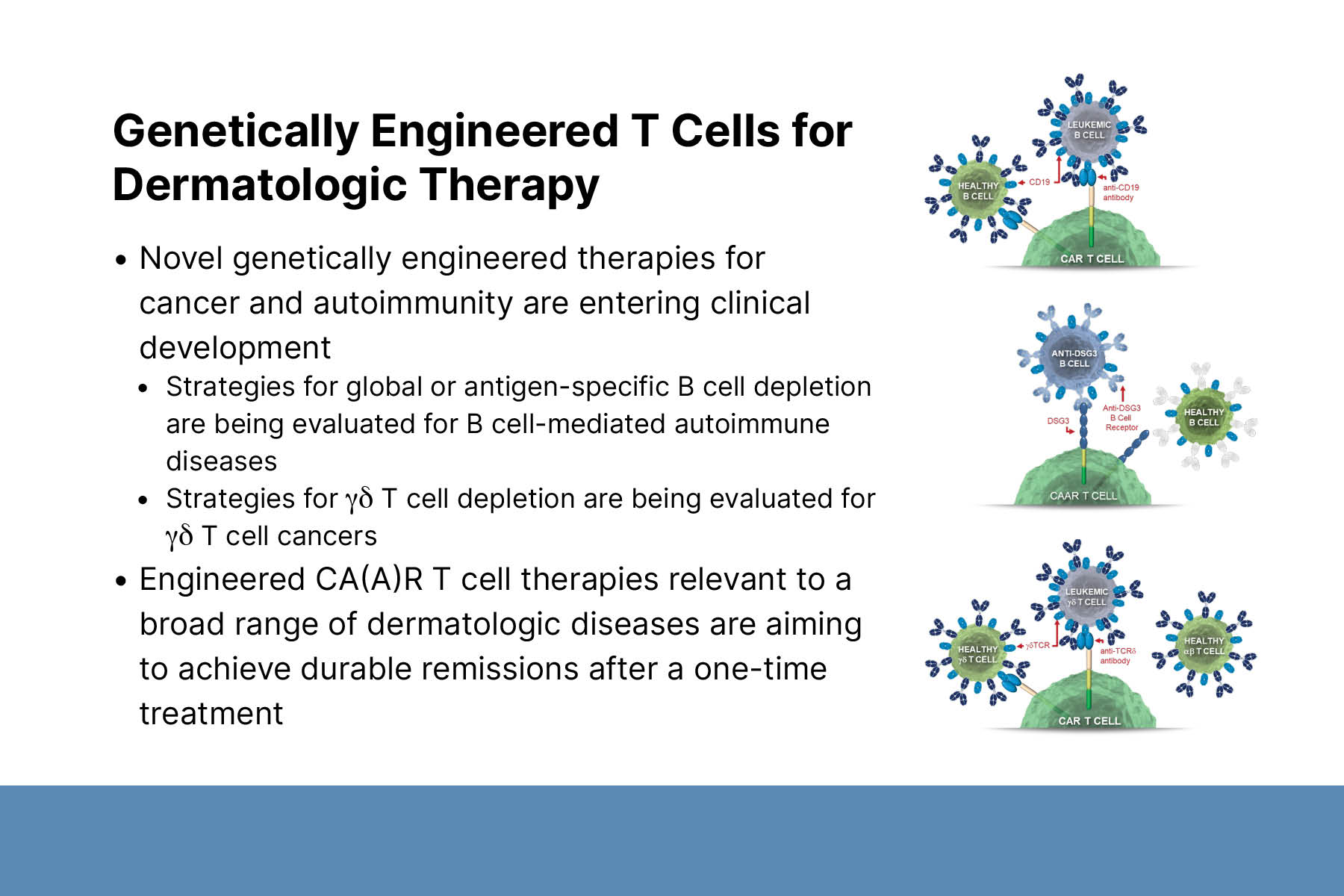
First, Dr. Payne described CAR-T cell therapy and its applications to cancer treatment. CAR-T cell therapy is a personalized immunotherapy technology. A patient’s T cells are collected, genetically altered to express chimeric antigen receptors, and reinfused. CAR-T cells seek out and kill target cells that express the specified antigen, such as CD19 or B cell maturation antigen (BCMA). Additionally, CAR-T cells are programmed to proliferate and produce memory CAR-T cells in the presence of target cells. There are 6 FDA-approved CAR-T cell immunotherapies for cancer, including CD19-CART for B cell leukemia and lymphoma and BCMA-CART for multiple myeloma. Treatment results in global B cell or plasma cell depletion, leading to increased risk of infection.
Second, Dr. Payne discussed PV, an autoimmune skin disease characterized by blisters on the skin and mucosal membranes (e.g., inner lining of the nose, mouth, esophagus and genitalia). It is caused by B cell-produced autoantibodies that attack the desmoglein 3 (DSG3) protein. Corticosteroids and rituximab can induce complete remission and reduce DSG3 autoantibodies to a normal range. However, maintenance regimens of rituximab are required to prevent disease relapse.
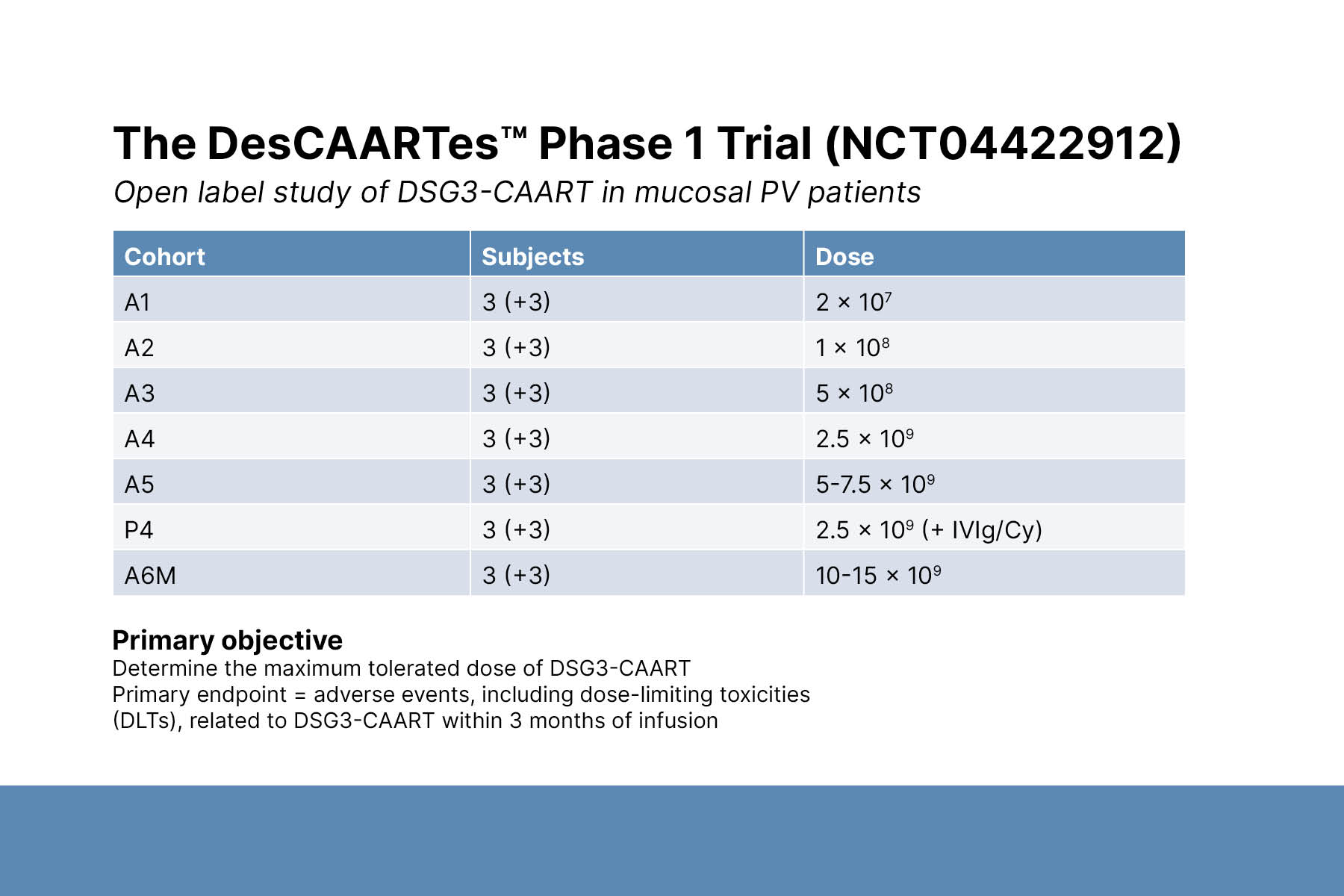
Third, Dr. Payne described the development of CAAR-T cell therapy for PV. DSG3-CAAR-T targets only B cells that produce DSG3 autoantibodies. Thus, it has the potential to treat disease without causing global B cell depletion. DSG3-CAAR-T is the first precision cellular immunotherapy to enter clinical trials for an autoimmune disease indication.
DSG3-CAAR-T was well tolerated and exhibited dose-dependent persistence across early dose cohorts in Phase 1 trials. Clinical improvements were observed in some patients. Ongoing cohorts are evaluating additional strategies, including pre-conditioning regimens and multiple infusions.
Fourth, Dr. Payne discussed additional applications of CAAR-T cell therapy for autoimmune diseases. MuSK-CAAR-T for muscle-specific tyrosine kinase (MuSK) myasthenia gravis recently entered clinical trials. Promising preclinical studies of PLA2R-CAAR-T targeting phospholipase A2 receptors (PLA2R) for membranous nephropathy will hopefully lead to clinical trials. Dr. Payne is collaborating with veterinarians to develop CAR-T cell therapy to treat autoimmune diseases in dogs.
Finally, Dr. Payne shared results from promising preclinical studies using CAR-T cell technologies to target gamma-delta T cell cancers that will hopefully lead to clinical trials.