JAK Inhibitor Therapy for Alopecia Areata
Crystal Aguh, MD
Associate Professor, Director, Ethnic Skin Program, Director, Ethnic Skin Fellowship, Department of Dermatology, Johns Hopkins School of Medicine
December 2023
Dr. Aguh presented about alopecia areata (AA) and treatment with JAK inhibitors.
First, Dr. Aguh described AA and the categories of disease severity. Common subtypes of AA include patch, totalis, and universalis; additional subtypes include ophiasis, incognito, and canitis subita, which manifests as sudden graying of the hair. She described that fortunately, most patients have relapsing-remitting disease and therefore in many cases will experience extended disease-free periods between flares. In ~25% of patients, the disease becomes progressively worse after initial presentation. Additionally, she noted that AA is associated with other autoimmune diseases and many patients will similarly report a family history of autoimmune diseases.
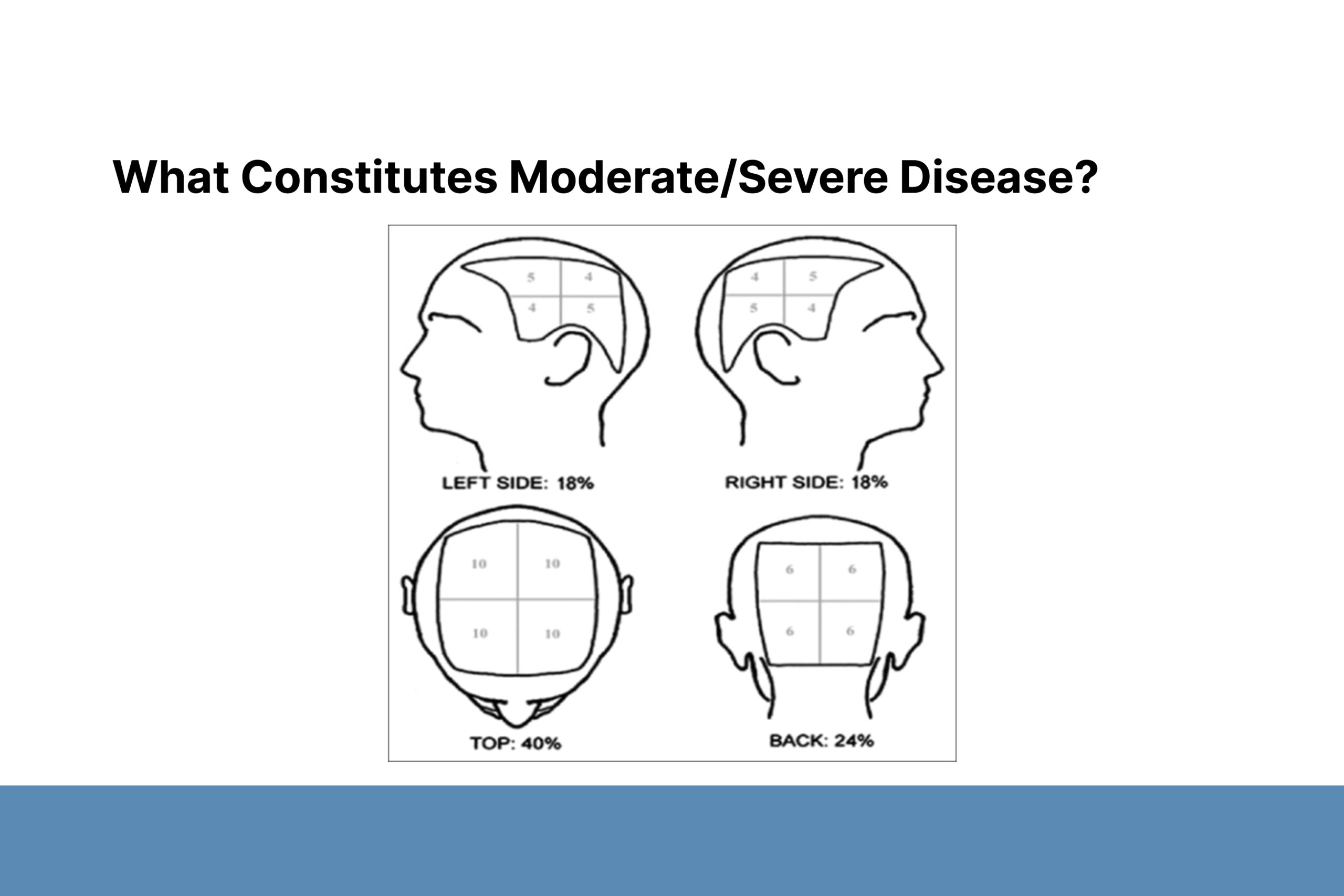
The severity of AA is determined using Severity of Alopecia Tool (SALT) scores, which are based on the percentage of scalp involvement: mild (≤20%), moderate (21–49%) or severe (≥50%) though this scale does not consider other important metrics physicians use when assessing patients. New methodology highlights additional features that can be associated with disease severity, including effects on quality of life (QoL), eyebrow or eyelash involvement, lack of response to standard treatment after 6 months, and rapidly progressing disease. Dr. Aguh emphasized that SALT score does not necessarily correlate with effects on QoL. For example, patients with short hair who are unable to cover active patches, may experience significant disfigurement even with limited disease
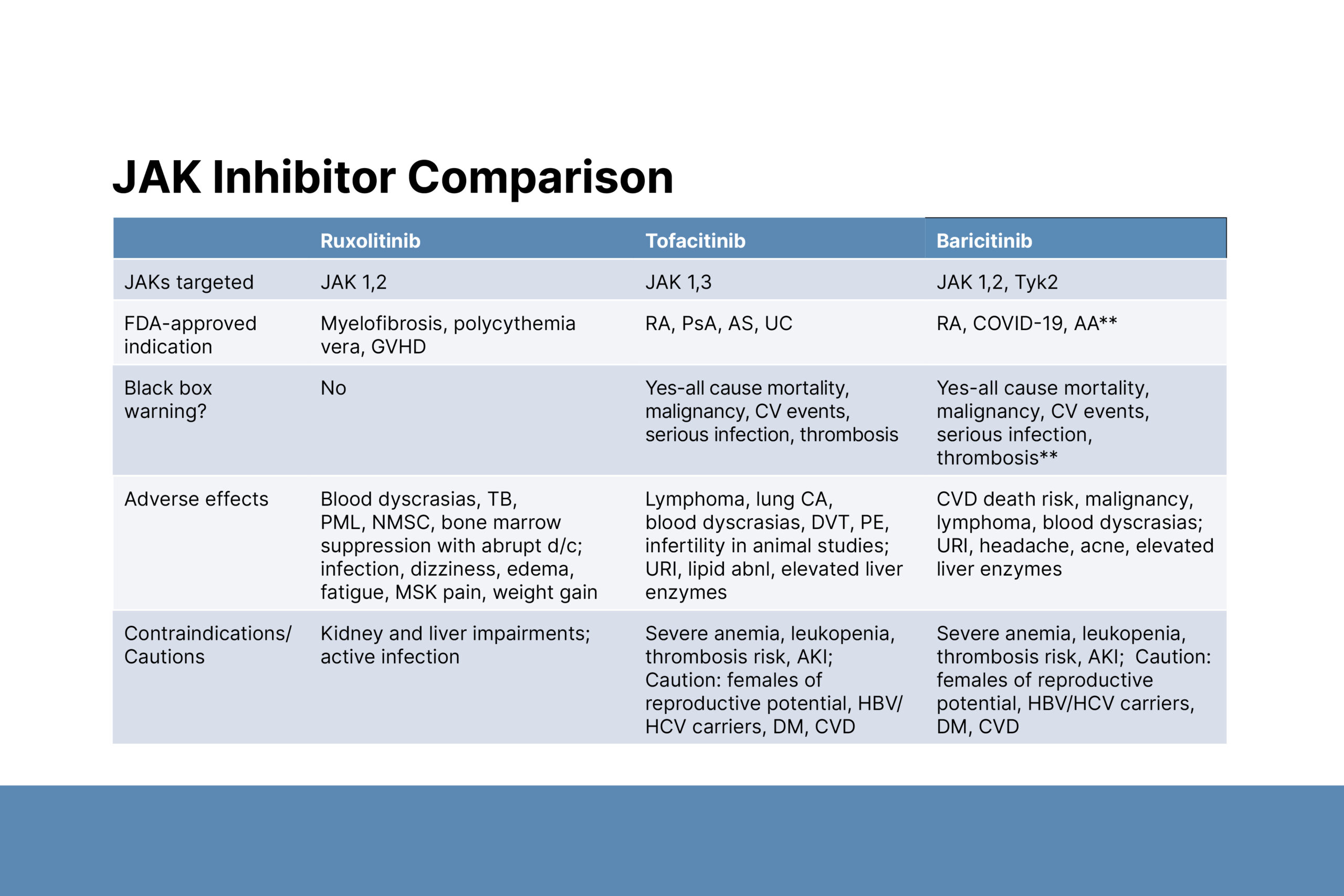
Second, Dr. Aguh described treatment options and the use of JAK inhibitors. First-line AA treatments include topical and intralesional steroids. More extensive disease is very difficult to treat and may include a combination of medications such as methotrexate, intramuscular Kenalog, minoxidil, and dupilumab. Considering the low response in patients with severe AA, alopecia totalis, and alopecia universalis, Dr. Aguh considers JAK inhibitors to be first-line medications in this group and has used several to treat her AA patients, including ruxolitinib, tofacitinib, and baricitinib. Baricitinib is the only JAK inhibitor that is FDA-approved for AA. Bloodwork must be monitored while patients are taking JAK inhibitors. Contraindications include kidney and liver impairments and blood disorders. Like tofacitinib and upadacitinib, baricitinib also carries a black box warning about the risk of blood clots, malignancy and an increase in all-cause mortality, with the latter warning being generated from data in patients taking tofacitiib.
Dr. Aguh emphasized the psychosocial effects of AA that warrant aggressive treatment like JAK inhibitors. Depression, anxiety, phobias, and panic disorders are more common in AA patients than in the general population. The negative effects on QoL are comparable to those associated with psoriasis and atopic dermatitis.
To close the session, Dr. Aguh shared JAK inhibitor dosing approaches for AA and prior authorization tips. Baricitinib is given at 2 mg for those with severe patch disease and 4 mg for universalis or totalis types. Other JAK inhibitors can be used off-label at the following doses: tofacitinib at 5 or 10 mg BID; ruxolitinib at 10 or 20 mg BID. The goal is to maintain the lowest effective dose. A dose increase is suggested if there is no AA improvement after 4–6 months of treatment. Currently, treatment duration is indefinite. For prior authorization, a SALT score ≥50 is helpful; listing prior therapies is helpful but not required. Patient assistance programs are available, and insurance coverage is improving.