Dr. Aimee Payne’s Path to Creating CAAR-T Cell Therapies
DF Stiefel Scholar Research Award
March 2024

In 2011, Aimee Payne, MD, PhD, was running a lab at the University of Pennsylvania, studying pemphigus vulgaris, an autoimmune disease of the skin that results when B cells make antibodies that attack the cell-adhesion protein desmoglein 3. The standard of care for pemphigus was corticosteroids and rituximab, a monoclonal antibody that targets the B-cell antigen CD20 and was originally approved for B-cell lymphoma. Meanwhile, across campus, Dr. Carl June and his team had just published a ground-breaking case series demonstrating long-term remission of refractory B-cell cancers with CAR-T cells that recognized the B-cell antigen CD19, work that led to the first FDA-approved CAR-T cell therapy, tisagenlecleucel (Porter et al. 2011).
“We were saying in the laboratory that anything that works for B-cell leukemia and lymphoma is going to work for pemphigus,” Dr. Payne said. “And all of a sudden, Carl June is reporting cures of B-cell lymphoma. After that, it seemed like every Penn faculty member was turning their favorite monoclonal antibody therapy into a CAR-T therapy. It was an incredibly exciting time, an ecosystem of collaboration and innovation.”
One of the postdoctoral fellows in her lab, Christoph Ellebrecht, MD (also a 2020 DF Dermatologist Investigator Research Fellowship awardee and recipient of a DF Career Development Award in 2021), who is now an assistant professor at Penn, spearheaded a collaboration with the laboratory of Michael Milone, MD, PhD, a co-inventor of tisagenlecleucel. That ultimately led to a further refinement of these innovations in a product known as chimeric autoantibody receptor (CAAR-T) cell therapy. CAAR-T cells are modified CAR-T cells designed to present an antigen recognized by only autoreactive B cells.
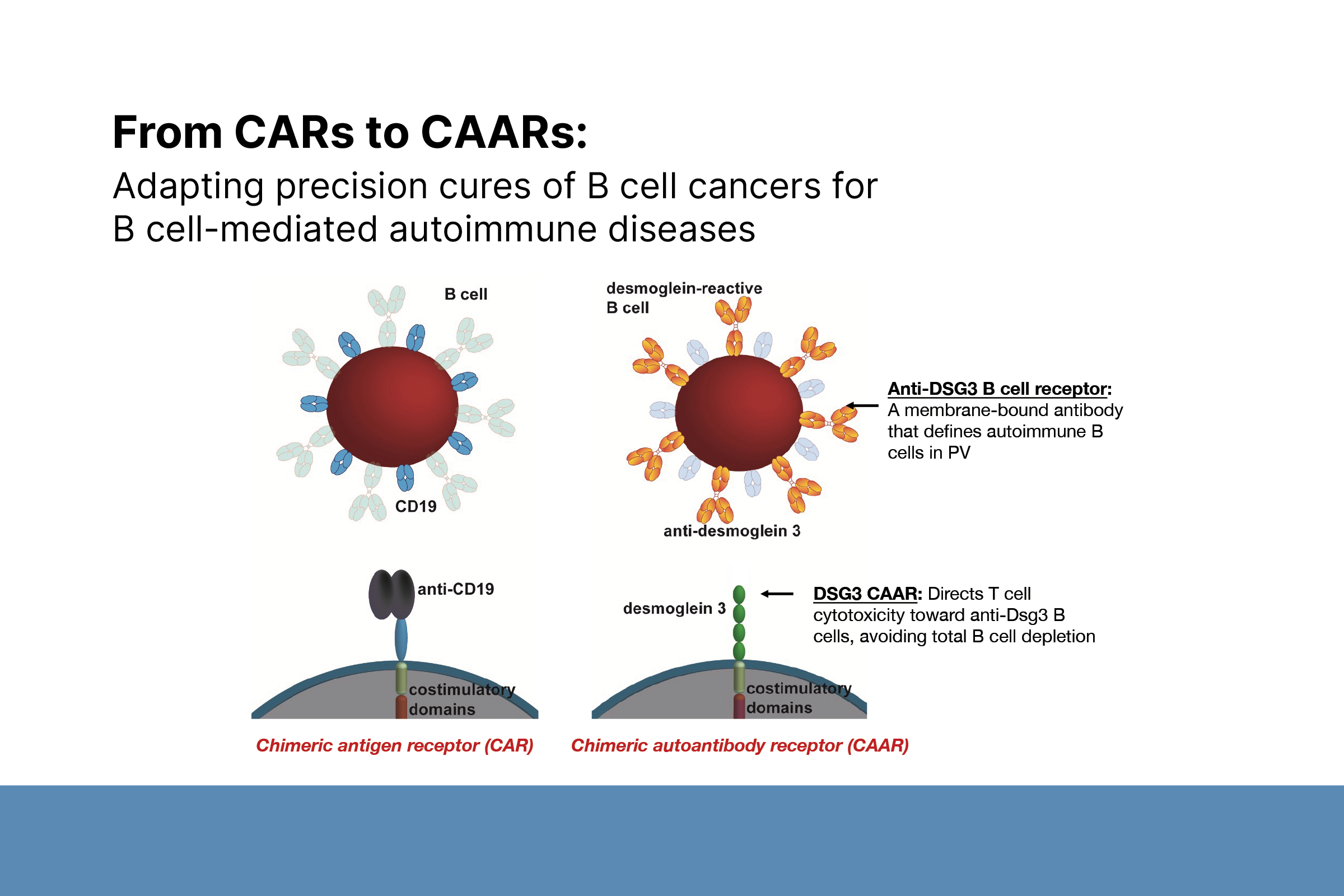
Adapting precision cures of B-cell cancers for B-cell-mediated autoimmune diseases. Photo courtesy of Christoph Ellebrecht, MD, 2020 Dermatologist Investigator Research Fellowship awardee and 2021 recipient of a DF Career Development Award in 2021.
“Our hope was that if CAAR-T cell therapy was as effective for B-cell mediated autoimmune diseases as CAR-T cell therapy was for B-cell mediated cancers, we could induce durable remissions of autoimmunity without the risks of global immunosuppression.” This led to promising preclinical studies of an experimental CAAR-T cell therapy for pemphigus called DSG3-CAART (Ellebrecht et al. 2016), in which the CAAR-T cells specifically target the DSG3 autoantibody producing B cells.
DF Charles and Daneen Stiefel Scholar Award
Designed to support outstanding mid-career investigators, the DF launched the Stiefel Scholar Research Award in 2014. Named after donors Charles and Daneen Stiefel, the award has enabled the DF to support three investigators whose exemplary research furthers the understanding of autoimmune and/or connective tissue diseases and three more investigators researching the treatment of skin cancer’s molecular and cellular basis.
Deep roots at Penn
Dr. Payne was at Penn for 22 years, as a resident, a postdoctoral fellow, and ultimately as a tenured Professor of Dermatology. She is president-elect of the Society for Investigative Dermatology (SID) and Co-Founder and Co-Chair of the Scientific Advisory Board of the biotech company, Cabaletta Bio. She became chair of the Department of Dermatology at Columbia University Vagelos College of Physicians and Surgeons and dermatologist-in-chief at New York-Presbyterian in December 2023.
Dr. Payne came to medicine through a love of basic science. She received her BS from Stanford University and her MD/PhD in molecular biology and cellular biology from Washington University School of Medicine. She was drawn to dermatology because almost every type of cell is found in skin, allowing a researcher to use it as a model to study a broad range of scientific disciplines such as molecular biology, cancer, immunology, neuroscience, or development.
“One of the appealing factors of working with pemphigus is that the autoantibodies are both necessary and sufficient to cause disease. I decided to use pemphigus as a model system to better understand how organ-specific autoimmunity occurs and how B cells develop autoreactivity to just this one protein.”
Dr. Payne used her background in dermatology and interest in autoimmunity, piqued during her residency, to explore the etiology of autoimmune skin disorders at the molecular level. She chose pemphigus because, unlike other autoimmune diseases in which the immune system goes awry more broadly (e.g., lupus), it is a focused immune attack on just one protein in the skin.
“One of the appealing factors of working with pemphigus is that the autoantibodies are both necessary and sufficient to cause disease,” she said. “I decided to use pemphigus as a model system to better understand how organ-specific autoimmunity occurs and how B cells develop autoreactivity to just this one protein. By figuring this out, we hope to develop better targeted therapies for disease as part of the trend toward precision medicines.”

Dr. Payne (foreground) with her team of CUIMC dermatology residents.
CAAR-T cell therapy for autoimmune skin diseases
DSG3-CAART leads to antigen-specific B-cell depletion by binding to the autoantibodies expressed on the surface of autoreactive B cells, also known as a B-cell receptor.
“It’s the perfect target because it basically declares that B cell as being autoreactive before it ever matures into an antibody-secreting cell.”
In a phase 1 dose escalation study, DSG3-CAART thus far has proven to have a tolerable safety profile, with some patients experiencing transient clinical improvements, though those have not been consistently associated with reductions in pemphigus antibody levels. The researchers are now exploring preconditioning regimens to see if they will enhance the response to DSG3 CAAR-T cells.
“Our hope was that CAAR-T therapy would be as effective as anti-CD19 CAR-T therapy is for B-cell cancers, requiring a one-time infusion leading to durable remission. Even if we find that the therapy is safe, effective and leads to disease control without the risk of global immunosuppression, it would be a substantial improvement to the current standard of care, in which patients often receive maintenance infusions of rituximab every six months.”
This could also sidestep the downside of rituximab treatments, which can be associated with increased risk for infections, and also can prevent effective vaccine responses.
Another experimental CAAR-T cell therapy developed in Dr. Payne’s lab is directed at muscle-specific tyrosine kinase (MuSK) myasthenia gravis. MuSK-CAART underwent preclinical testing in an animal model (Oh et al. 2023) and is now in a phase 1 clinical trial at several sites around the country.
Another experimental CAAR-T cell therapy developed in Dr. Payne’s lab is directed at muscle-specific tyrosine kinase (MuSK) myasthenia gravis. MuSK-CAART underwent preclinical testing in an animal model (Oh et al. 2023) and is now in a phase 1 clinical trial at several sites around the country.
“The MuSK-CAART treatment is of interest because we believe those patients may have up to ten-fold lower soluble antibody levels in circulation compared to pemphigus vulgaris patients,” Dr. Payne said. “This makes it an interesting study to determine whether the soluble antibody levels and, more specifically, the reduction in those levels, plays a role in the effectiveness of the therapy.”
Other preclinical research is looking at PLA2R-CAART, which recognizes phospholipase A2 receptors (PLA2R) on autoreactive B cells and is a potential treatment for membranous nephropathy. Dr. Payne is also exploring veterinary applications for CAAR-T cell therapy. Since dogs naturally get pemphigus and many other autoimmune diseases that afflict humans, she collaborates with Nicola Mason, PhD, at the University of Pennsylvania, to study dogs as a model to understand human autoimmune disease and cancer therapy.
Combining academia and entrepreneurship
After she published their proof-of-concept paper in 2016, Dr. Payne wondered how to advance that work to clinical trials. She knew the cost of the next steps—an investigational new drug (IND) application, clinical trials, regulatory approval, and GMP manufacturing—would far exceed typical financial support available through federal grants. Dr. Payne co-founded Cabaletta Bio to advance these promising cellular immunotherapies for autoimmunity to clinical trials. She has found that helping run a biotechnology company in the midst of her academic career has been a great education to learn how medicines in the US are developed and approved. Launching Cabaletta Bio was a way to gather the funds to support this translational work, while also providing the clinical and regulatory expertise to advance the science.
“When I was an MD/PhD student, someone said that nothing we would do in the laboratory would reach a patient in our lifetime because of the time it took to discover, develop, and clinically test a new therapy,” she said.
That’s no longer true. For tisagenlecleucel (Kymriah), only six years elapsed between the original case studies and FDA approval for the treatment of acute lymphoblastic leukemia.
“It’s been incredibly synergistic to be involved in both business and research. Entrepreneurship has sharpened the translational focus and rationale for the type of scientific questions we ask in the laboratory.”
Whether a young student is interested in academia or business, Dr. Payne believes investigative dermatology is an ideal choice for a physician-scientist. “There are so many possible career paths, whether in clinical care, education, research, government, or drug development. You’re only limited by your imagination and passion for pursuing that path. You can mix and match those to basically design your career of choice.”
Crucial DF support
In 2006, Dr. Payne received a physician scientist Career Development Award and basic research funding from the Foundation. She was also the recipient of a Stiefel Scholar Award in 2015, which recognizes exceptional mid-career researchers studying autoimmune and connective tissue diseases and the treatment of skin cancers at the cellular and molecular level.
“My message to donors is, thank you for paying it forward. It’s a great investment in the future of our specialty and a way for dermatology to retain its place as a key contributor to the house of medicine.”
“My message to donors is, thank you for paying it forward. It’s a great investment in the future of our specialty and a way for dermatology to retain its place as a key contributor to the house of medicine.”
“I’m incredibly grateful to the Foundation,” she said. “Both these awards occurred during critical times in my career. In 2006, the awards ensured continued research funding that allowed me to receive my tenure track faculty appointment. Similarly in 2015, the Stiefel Scholar Award bolstered my tenure application and led to the great honor of becoming a tenured faculty member at the University of Pennsylvania.
“The Foundation has a unique niche in our specialty because it encourages physicians and others to give back to our specialty. The Research Award Program has helped so many outstanding young dermatologists and skin researchers, not only financially, but through the professional and scientific mentorship that it affords.”
Biography
Aimee Payne, MD, PhD is chair of the Department of Dermatology at Columbia University Vagelos College of Physicians and Surgeons and dermatologist-in-chief at New York-Presbyterian.
She has served as chair of the National Institute of Arthritis and Musculoskeletal and Skin Diseases Board of Scientific Counselors and is Co-founder and Co-chair of the Scientific Advisory Board of Cabaletta Bio, a publicly traded biotechnology company that focuses on engineering cellular immunotherapies for autoimmune diseases.
She is an elected member of the American Society for Clinical Investigation and the American Dermatological Association and serves on the Medical Advisory Council of the International Pemphigus and Pemphigoid Foundation. Dr. Payne received her bachelor’s degree in biology from Stanford University and is a graduate of the Washington University School of Medicine in St. Louis, where she earned her MD and a PhD in molecular and cellular biology. She completed her dermatology residency and postdoctoral fellowship at the University of Pennsylvania.
Selected publications
Oh S, Mao X, Manfredo-Vieira S, Lee J, Patel D, Choi EJ, Alvarado A, Cottman-Thomas E, Maseda D, Tsao PY, Ellebrecht CT, Khella SL, Richman DP, O’Connor KC, Herzberg U, Binder GK, Milone MC, Basu S, Payne AS: Precision targeting of autoantigen-specific B-cells in muscle-specific tyrosine kinase myasthenia gravis with chimeric autoantibody receptor T-cells. Nature Biotechnol, 41L1229–1238, 2023.
Tovanabutra N, Bax CE, Feng R, Kushner CJ, Payne AS: Temporal outcomes after rituximab therapy for pemphigus. J. Invest. Dermatol. 142(4): 1058-1064, 2022 Notes: doi: 10.1016/j.jid.2021.09.013.
Lee J, Lundgren DK, Mao X, Manfredo-Vieira S, Nunez-Cruz S, Williams EF, Assenmacher CA, Radaelli E, Oh S, Wang B, Ellebrecht CT, Fraietta JA, Milone MC, Payne AS: Antigen specific B-cell depletion for precision therapy of mucosal pemphigus vulgaris. J Clin Invest 130(12): 6317-6324, 2020 Notes: doi:10.1172/JCI13841.
References
Ellebrecht CT, Bhoj VG, Nace A, et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science. 2016;353(6295):179–184.
Oh S, Mao X, Manfredo-Vieira S, et al. Precision targeting of autoantigen-specific B cells in muscle-specific tyrosine kinase myasthenia gravis with chimeric autoantibody receptor T cells. Nat Biotechnol. 2023;41(9):1229–1238.
Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. Erratum in: N Engl J Med. 2016;374(10):998.
Learn more about the DF Research Awards.

