Pragmatic Trials: The Next Generation of Research Needed to Advance Dermatology Practice
July 2025
Dr. Joel Gelfand presented information about real-world data collection methods, including registries, automated databases, and pragmatic clinical trials.
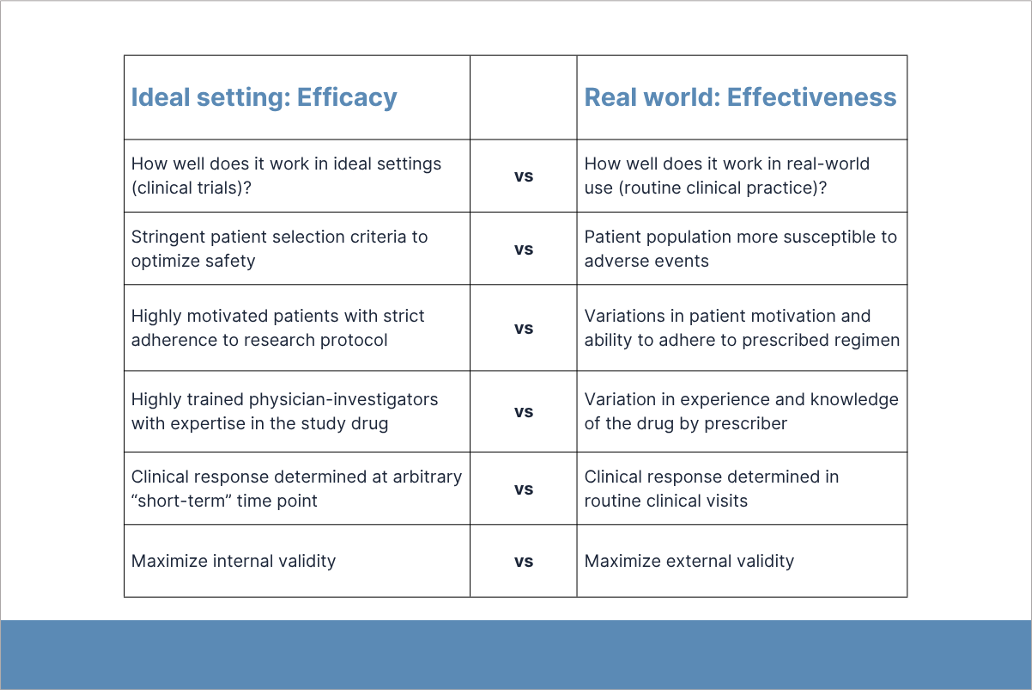
First, Gelfand discussed the role of real-world studies in identifying adverse events (AEs) caused by newly approved treatments. Pre-approval clinical trials (i.e., phase 1-3) have limited ability to identify safety signals because these studies are designed to optimize efficacy and safety outcomes, have short-term exposure to the intervention medication, and lack sufficient statistical power. The patient populations in these studies are not large enough to detect rare but serious AEs (e.g., thrombosis, severe infection). Real-world studies monitor the effectiveness and safety of new treatments after FDA approval. The FDA’s real-world evidence program includes MEDWATCH and the Sentinel Initiative.
Pragmatic clinical trials help show how treatments actually work in everyday care. Done after FDA approval, they test effectiveness in real-world settings and still use randomization to keep the results reliable.
Clinical dermatologists should know that it may take 4-5 years after FDA approval to fully understand the safety profiles of new agents. About 33% of newly approved drugs get Black Box warnings added to their product labeling after approval; 1.3% are withdrawn. Biologics and psychiatric therapeutics have the highest rates of new safety warnings added to their labels after approval. Gelfand shared examples of real-world studies that identified post-market safety issues.
Gelfand cautioned dermatologists to carefully consider the results of real-world studies, especially disproportionality analyses of spontaneous reports. Disproportionality analyses have increased exponentially since the FDA made their AE reporting system publicly available in 2018, but they are prone to systematic errors.
Second, Gelfand described the strengths and limitations of registries and automated databases for real-world data collection. Registries collect and store clinical data in a standardized format. They provide detailed data on variables (e.g., disease severity; patient-reported outcomes) that are not measured by automated databases. However, they are prone to selection and information bias.
Automated databases (e.g., administrative claims and medical records) minimize selection and information bias by capturing all patients and outcomes, improve internal and external validity, and increase statistical power with larger sample sizes. However, these data must be carefully validated to avoid systematic errors. Gelfand emphasized that observational studies using registries and automated databases are often insufficient to establish causality.
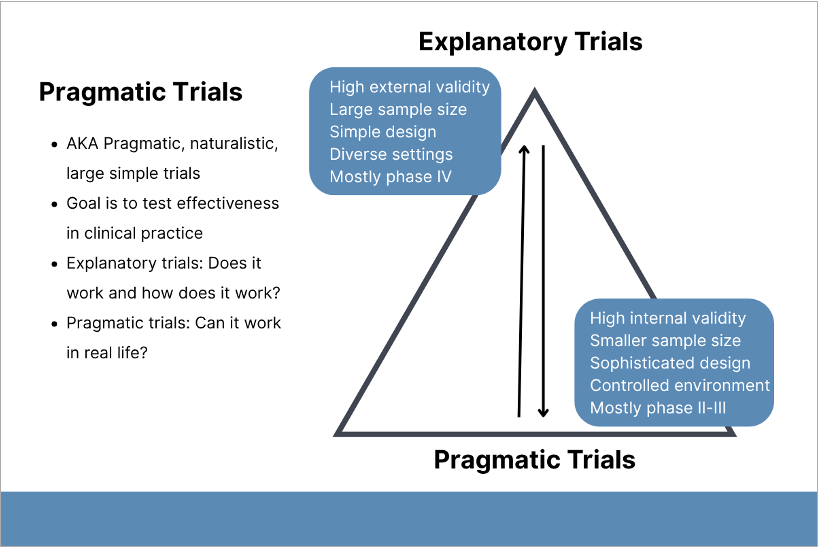
The wide base of the pyramid depicts the relatively higher proportion of explanatory trials. Creative Commons License under CC BY
Third, Gelfand discussed the advantages of pragmatic clinical trials. These studies are conducted after FDA approval and designed to evaluate the effectiveness of a treatment in real-world clinical practice where patients can still be randomized. Compared to participants in pre-approval clinical trials, patients in real-world studies may be sicker, have other health issues, and/or be less adherent to treatment.
Gelfand shared information about the study design and results of LITE, a randomized pragmatic clinical trial that evaluated home versus office-based narrowband ultraviolet-B phototherapy for patients with psoriasis. The patient population was representative of those with moderate-to-severe psoriasis enrolled in pre-approval clinical trials for systemic or biologic agents, with fewer exclusion criteria and more women, adolescents, and all skin types compared to traditional efficacy trials used to obtain FDA approval of a drug or biologic.
To conclude, Gelfand encouraged dermatologists to participate in pragmatic clinical trials and report serious or unusual drug reactions (e.g., toxic epidermal necrolysis, Stevens-Johnson syndrome) to the FDA or manufacturer.
Mark January 28-31, 2026 on your calendar for the 2026 Annual DF Clinical Symposium.

